CHICAGO – A five-minute, point-of-care coronavirus test could be coming to hospitals next week, and experts say it could be "game-changing."
The U.S. Food and Drug Administration issued Emergency Use Authorization to Illinois-based medical device maker Abbott Labs on Friday for a coronavirus test that delivers positive results in as little as five minutes and negative results in 13 minutes, the company said.
The company expects the tests to be available next week and expects to ramp up manufacturing to deliver 50,000 tests per day, according to a press release.
"I am pleased that the FDA authorized Abbott's point of care test yesterday. This is big news and will help get more of these tests out in the field rapidly," said FDA Commissioner Steve Hahn in a statement. "We know how important it is to get point of care tests out in the field quickly. These tests that can give results quickly can be a game changer in diagnosing COVID-19."
Get daily coronavirus updates in your inbox: Sign up for our newsletter now.
8 strains of the coronavirus are circling the globe: Here's what clues they're giving scientists.
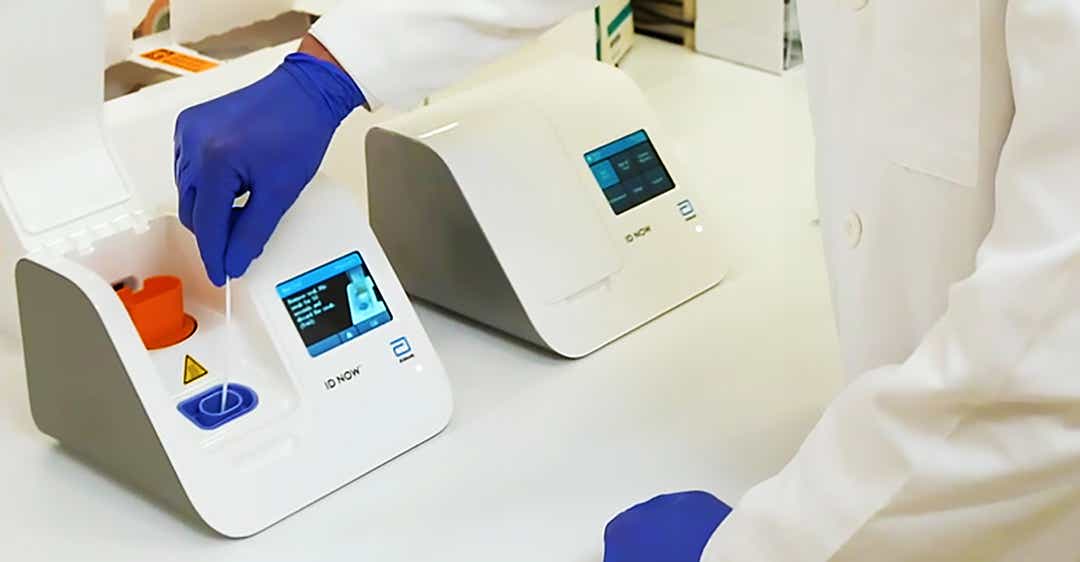
According to Abbott, the test will run on the company's ID NOW platform, a 6.6-pound portable device the size of a small toaster. The ID NOW is the most widely available molecular point-of-care testing platform in the U.S. today, according to Abbott.
Last week, the FDA approved another coronavirus test from Abbott, the m2000 RealTime SARS CoV-2 EUA test. Between the two platforms, Abbott expects to produce about 5 million tests per month.
Scott Gottlieb, former FDA commissioner, echoed Hahn's comments on Twitter, calling the development a "game changer."
Gottlieb also said it’s "very likely" that we’ll see additional approvals of point-of-care diagnostics behind this one, extending testing to doctor offices across the U.S.
The U.S. has struggled to ramp us testing across the nation, and, in the early days of the outbreak, the Centers for Disease Control and Prevention initially developed and mailed flawed testing kits. Many doctors have reported rationing tests to prioritize the most vulnerable.
Public health labs, as well as approved private labs, universities and hospitals, can conduct the tests. As of March 26, 92 public health laboratories in 50 states plus Washington D.C., Guam and Puerto Rico had successfully verified COVID-19 diagnostic tests and were offering testing, according to the CDC.
The coronavirus test that wasn't: How federal health officials misled state scientists and derailed the best chance at containment

While there are no official nationwide testing totals, the COVID Tracking Project, an online effort started by two journalists to track the number of tests conducted in the U.S., said Friday that more than 620,000 tests had been completed nationwide – the most tests conducted by any country.
But the U.S., with a population of about 329 million, is not leading the world in the number of tests conducted per capita. South Korea, a country of about 51 million, had tested more than 370,000 people as of Friday, according to health officials. Italy, which has a population of 60 million and has reported the most coronavirus-related deaths, had tested more than 390,000 people, according to health officials.
Dr. Deborah Birx, the White House's coronavirus response coordinator, said in a Friday briefing that about 95% of testing in the U.S. is being done in commercial labs. The $2.2 trillion stimulus package approved Friday will require those labs to report total testing numbers, Birx said.
Follow Grace Hauck on Twitter @grace_hauck.
https://news.google.com/__i/rss/rd/articles/CBMigQFodHRwczovL3d3dy51c2F0b2RheS5jb20vc3RvcnkvbmV3cy9oZWFsdGgvMjAyMC8wMy8yOC9jb3JvbmF2aXJ1cy1mZGEtYXV0aG9yaXplcy1hYmJvdHQtbGFicy1mYXN0LXBvcnRhYmxlLWNvdmlkLXRlc3QvMjkzMjc2NjAwMS_SASdodHRwczovL2FtcC51c2F0b2RheS5jb20vYW1wLzI5MzI3NjYwMDE?oc=5
2020-03-28 16:25:59Z
52780693412014
Tidak ada komentar:
Posting Komentar